Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC | Nature Reviews Clinical Oncology

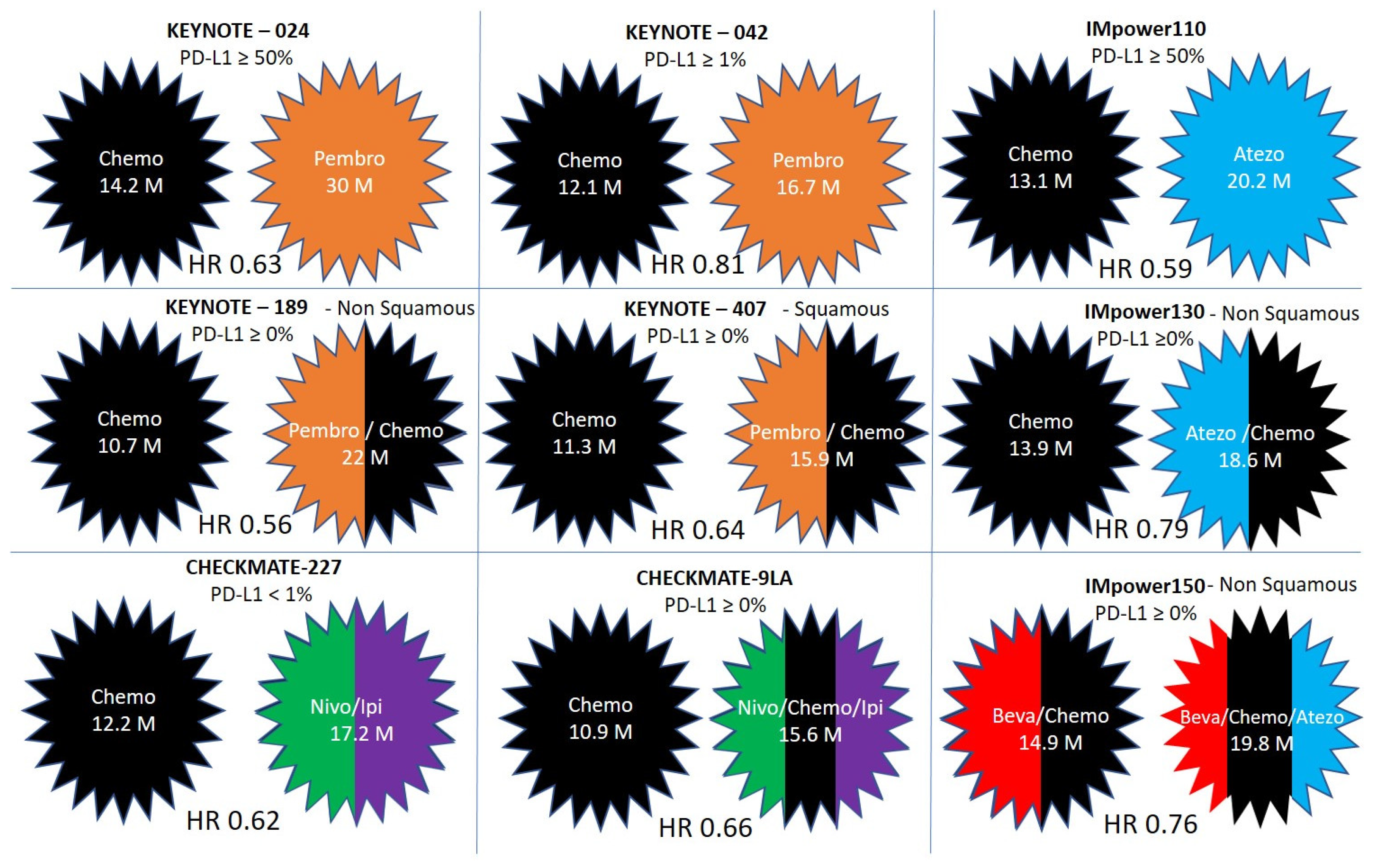
Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial - The Lancet
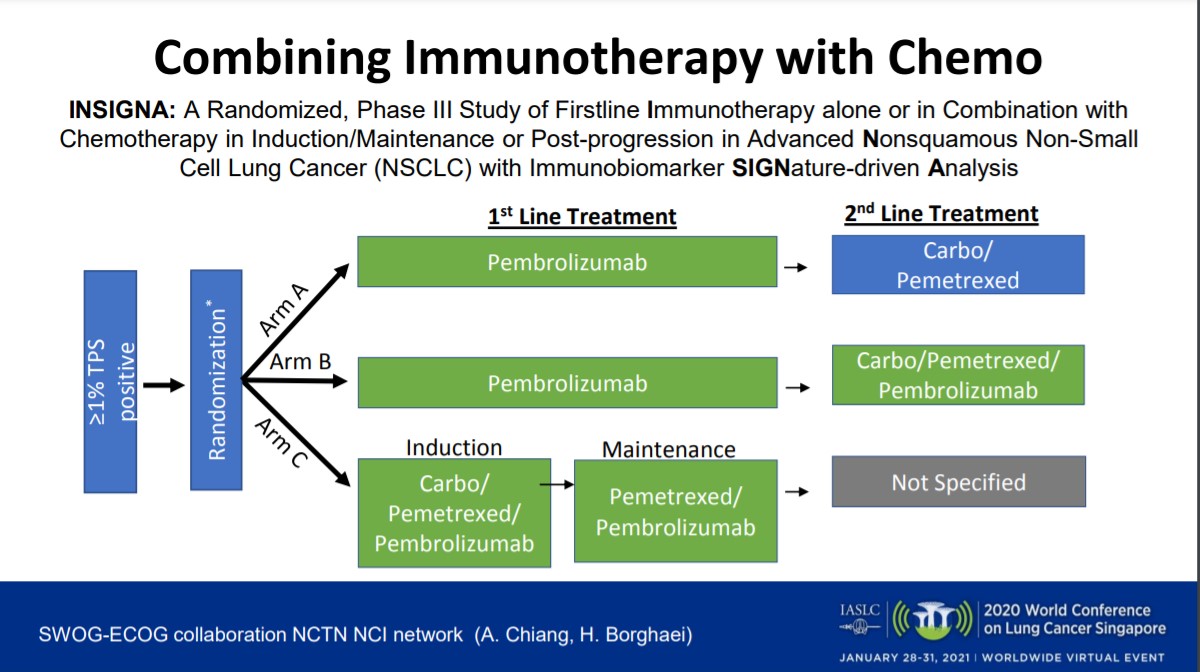
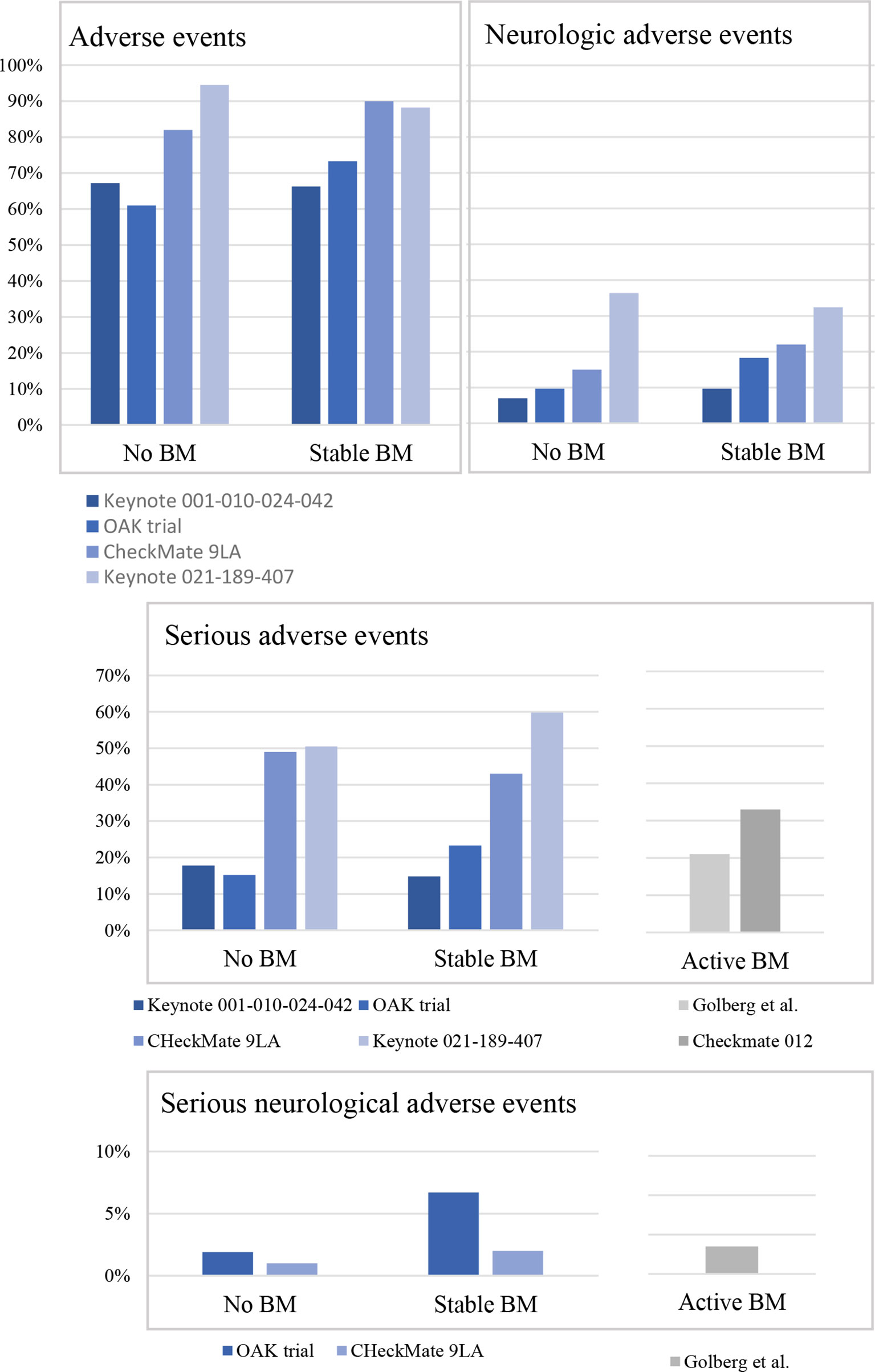
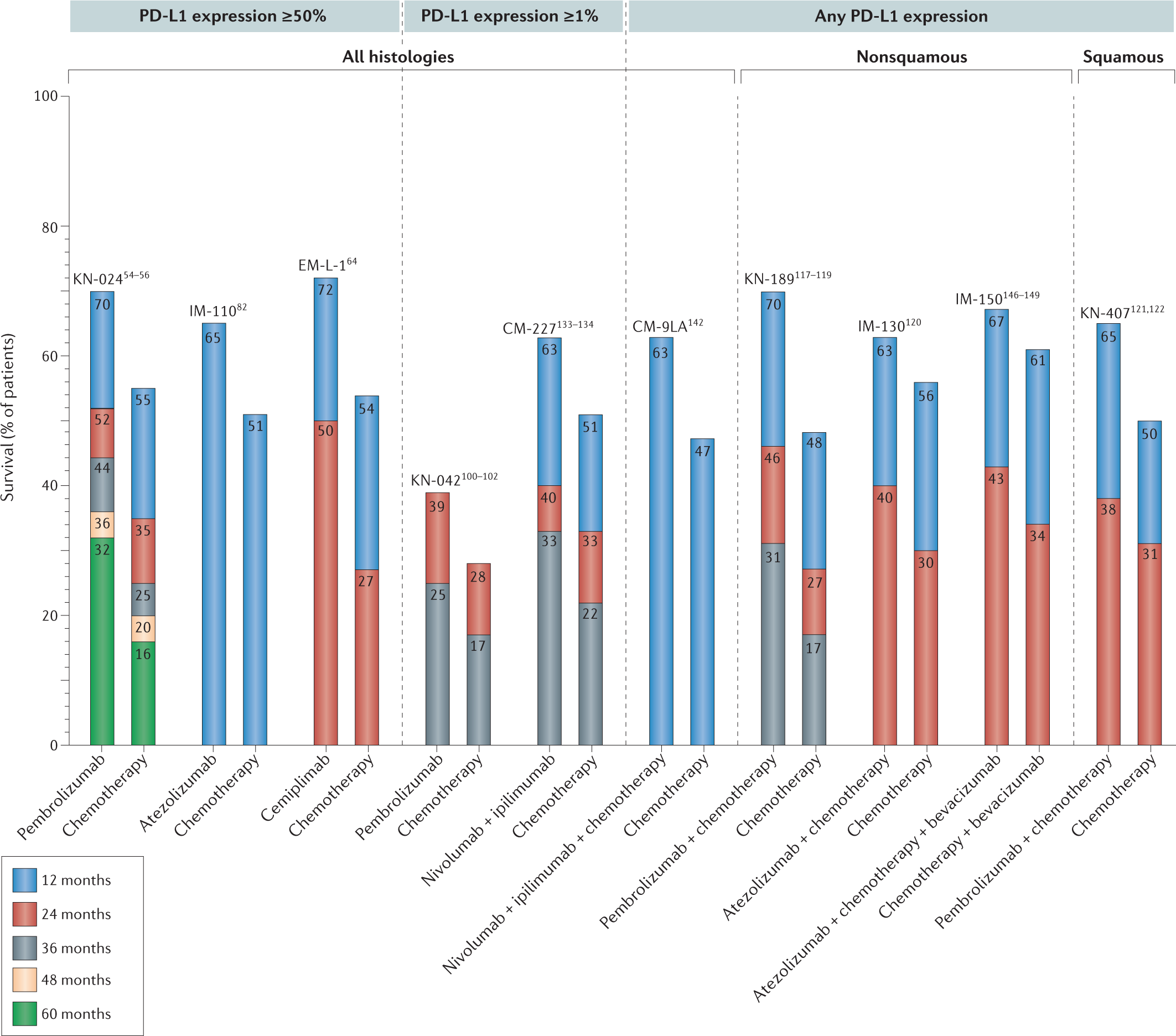
Use of Predictive Biomarkers, Incorporating Chemotherapy—What Is Best When Using Immunotherapy in Advanced NSCLC? - ILCN.org (ILCN/WCLC)

Study characteristics for CheckMate 227 Part 1A, KEYNOTE-021 Cohort G,... | Download Scientific Diagram

Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial - The Lancet
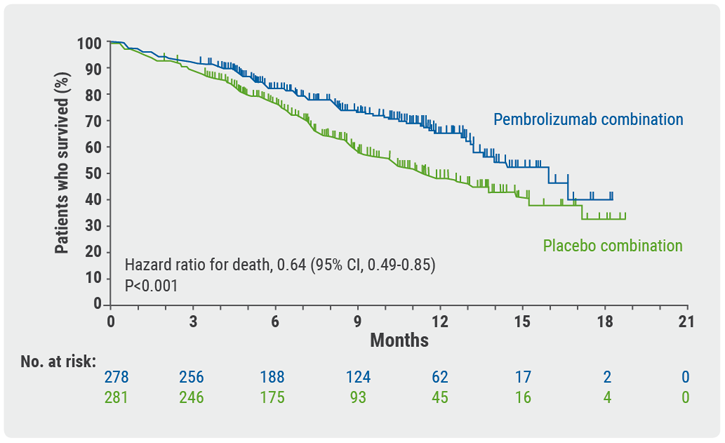
Choice of taxane and addition of pembrolizumab for metastatic squamous non-small-cell lung cancer - Medical Conferences

Pembrolizumab plus chemotherapy versus chemotherapy alone in patients with advanced non–small cell lung cancer without tumor PD‐L1 expression: A pooled analysis of 3 randomized controlled trials - Borghaei - 2020 - Cancer -

Immunotherapy Succeeds in Squamous NSCLC, Establishing a New Frontline Standard of Care - ILCN.org (ILCN/WCLC)

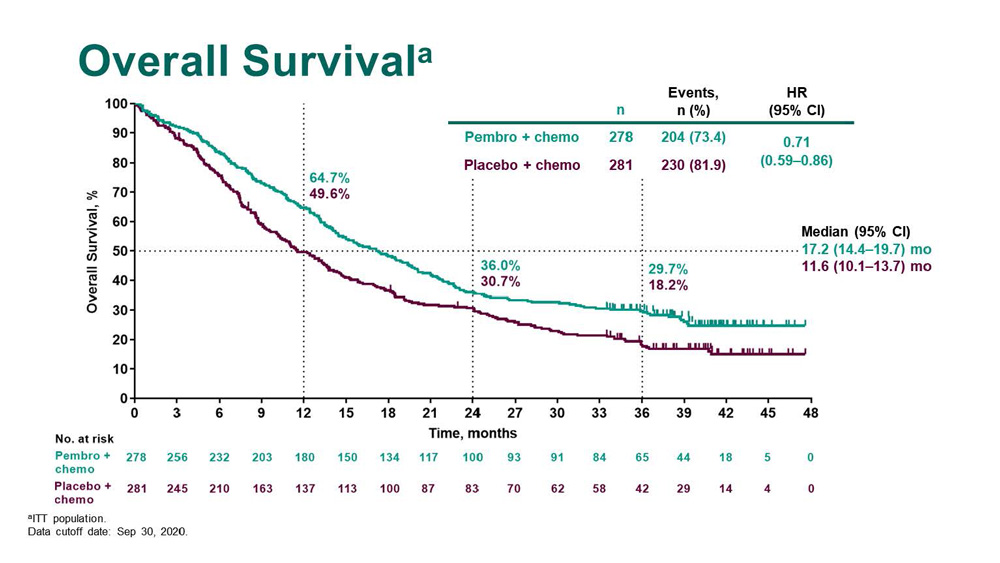
A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407 - Journal of Thoracic Oncology

Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results From a Randomized Phase III Trial - Journal of Thoracic Oncology

ICIs in NSCLC - NSCLC Treatment Paradigm - Text Module - NSCLC Practical Guidance - Oncology - Clinical Care Options